Membraneless compartments--models for a potential step in the early evolution of cells--have been shown to persist or form, disappear, and reform in predictable ways through multiple cycles of dehydration and rehydration. Such wet-dry cycles were likely common conditions during the early development of life on Earth and could be a driving force for reactions important for the evolution of life.
Understanding how the compartments--known as complex coacervates--respond to wet-dry cycling also informs current applications of the droplets, which are found in many household items, such as adhesives, cosmetics, fragrances, and food, and could be used in drug delivery systems. A paper describing the research, led by Penn State scientists, appears in the journal Nature Communications.
"Wet-dry cycling has gotten attention recently in attempts to produce molecules that could be the precursors to life, things like the building blocks of RNA, DNA, and proteins," said Hadi Fares, a NASA Postdoctoral Program Fellow at Penn State and the first author of the paper. "We are looking into a possible step further in the evolution of life. If these building blocks form compartments--the precursors of cells--what happens if they undergo the same type of wet-dry cycling?"
The researchers make membraneless compartments, which form through liquid-liquid phase separation in a manner akin to oil droplets forming as a salad dressing separates, by controlling the concentrations of reagents in a solution. When the conditions--pH, temperature, salt and polymer concentrations--are right, droplets form that contain higher concentrations of the polymers than the surrounding solution. Like oil drops in water, there is no physical barrier or membrane that separates the droplets from their surroundings.
Dehydrating the solution, like what could happen during dry periods on a pre-life Earth where small ponds or puddles might regularly dry up, changes all of these factors. The researchers, therefore, wanted to know what would happen to the membraneless compartments in their experimental system if they recreated these wet-dry cycles.
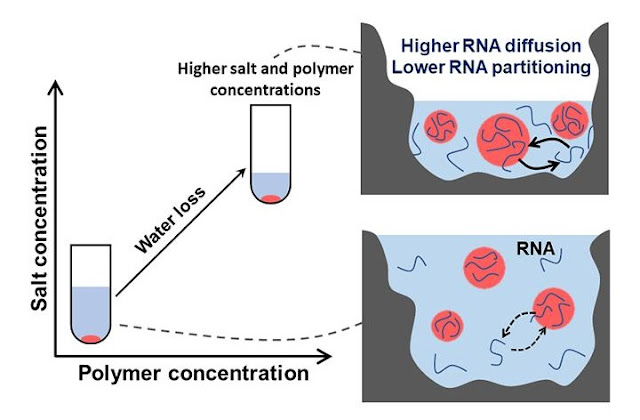
"We first mapped out how the compartments form when we alter the concentrations of the polymers and the salt," said Fares. "This 'phase diagram' is experimentally determined and represents the physical chemistry of the system. So, we know whether or not droplets will form for different concentrations of polymers and salt. We can then start with a solution with concentrations at any point on this phase diagram and see what happens when we dehydrate the sample."
If the researchers start with a solution with concentrations that favor the formation of droplets, dehydration can change the concentrations such that the droplets disappear. The droplets then reappear when the sample is rehydrated. They can also start with a solution in which no droplets form and dehydration could bring the concentrations into the range that droplets begin to form. The behavior of the droplets during dehydration and rehydration match the predictions based on the experimentally derived phase diagram and they continue to do so through several iterations of the wet-dry cycle.
Next, the researchers addressed the ability of droplets to incorporate RNA molecules inside of the membraneless compartments. The "RNA world" hypothesis suggests that RNA may have played an important role in the early evolution of life on Earth and previous experimental work has shown that RNA in these solutions becomes concentrated inside of the droplets.
"As we dry droplets that contain RNA, the overall concentration of RNA in the solution increases but the concentration of RNA inside the droplets remains fairly stable," said Fares. "The preference of RNA molecules to be inside the droplets seems to decrease. We believe that this is because as they dry the composition inside the droplets is changing to look more like the composition outside the droplets."
The research team also looked at the ability of RNA to move into and within the droplets during dehydration. As they dry the sample the movement of RNA into and out of the droplets increases massively, but movement within the droplets increases only modestly. This difference in RNA mobility could have implications for the exchange of RNA among droplets during dehydration, which could in turn be functionally important in protocells.
"What we are showing is that as the membraneless compartments dry, they are able to preserve, at least to some extent, their internal environment," said Fares. "Importantly, the behavior of the coacervates, or protocells, whether they persist or disappear and reappear through the wet-dry cycle, is predicable from the physical chemistry of the system. We can therefore use this model system to think about the chemistry that might have been important for the early evolution of life."
Beyond early life scenarios, the research has implications much closer to home.
"People underestimate how important coacervates are beyond their role as a model for protocells," said Christine Keating, Distinguished Professor of Chemistry at Penn State and leader of the research team. "Many of the things that you have in your house that appear cloudy have coacervates in them. Any time you want to compartmentalize something, whether it's for drug delivery, a fragrance, a nutrient, or food product, coacervates may be involved. Understanding something new about the physical chemistry of the process of droplet formation will be important for all of these things."
Author: Sam Sholtis | Source: Pennsylvania State University [October 28, 2020]

No comments:
Post a Comment